Ref: A. Ma, M. Dong, J. Cheng et al, Journal of Intensive Medicine 3 (2023) 65–72, https://doi.org/10.1016/j.jointm.2022.05.006
The intensive care unit (ICU) is the “hardest hit” department for hospital-acquired infections, where a range of traumatic procedures can lead to fatal infections. Ventilator-related pneumonia, catheter-related bloodstream infections caused by an arterial or venous puncture, and skin and soft tissue infections (SSTIs) secondary to tracheotomy and surgical wounds are the main types of hospital-acquired infections. Nearly half of these infections are caused by Gram-positive bacteria. The increasing prevalence of multidrug-resistant Gram positive pathogens poses a significant challenge in the ICU. Methicillin-resistant Staphylococcus aureus (MRSA), methicillin susceptible Staphylococcus aureus (MSSA), and vancomycin resistant Enterococci (VRE), which are extremely common in the ICU, are regarded as priority pathogens that cause morbidity and mortality in countless cases. Therefore, it is essential for ICU clinicians to identify resistance patterns in Gram-positive bacteria and use antibiotics that are effective against these resistant phenotypes. Linezolid, a synthetic oxazolidinone antibiotic, has been approved for the treatment of infections caused by VRE, hospital acquired pneumonia caused by MRSA and MSSA, complicated SSTIs caused by Staphylococcus aureus or Streptococcus pneumoniae, and uncomplicated SSTIs caused by Staphylococcus aureus (methicillin-resistant only) or Streptococcus pyogenes. The drug has favorable in vitro and in vivo activity against the mentioned organismsy.
As advanced antibiotics commonly used in the ICU, linezolid and vancomycin are often compared. Although vancomycin is often used as the first choice, side effects pertaining to liver and kidney function, and especially renal injury, lead to certain limitations in its use in ICU patients. Linezolid is more useful than vancomycin in SSTIs and MRSA pneumonia, and is more effective and cost-effective for hospital-acquired MRSA infections. It is also the only antibiotic that is more effective than daptomycin and quinopidine (among others) for vancomycin resistant Enterococcus faecalis infection. Considering the role of linezolid in the treatment of Gram positive bacteria, this real-world study was conducted to characterize the population of critically ill patients in the ICU and infections treated with linezolid in the ICU. It also aimed to evaluate the clinical efficacy of linezolid therapy and to assess the safety of Chinese-made linezolid in ICU patients. This study collected data from 52 hospitals and conducted a retrospective analysis to guide better antibiotic prescribing among clinicians.
Clinical outcome by infecting pathogen. MRSA: Methicillin-resistant Staphylococcus aureus; MSSA: Methicillin-susceptible Staphylococcus aureus; SSTI: Skin and soft tissue infections; VRE: Vancomycin-resistant Enterococci; VSE: Vancomycin-susceptible Enterococci.
Methods:
This multi-center, observational, real-world study was conducted across 52 hospitals between June 9, 2018, and December 28, 2019. Patients who met the following inclusion criteria were included: (1) admitted to the ICU, (2) of any age group, and (3) having a clinical or laboratory diagnosis of a Gram-positive bacterial infection. Clinical efficacy was categorized as success (cured or improved), failed, or non-evaluable. Adverse events and serious adverse events were recorded during treatment.
Results:
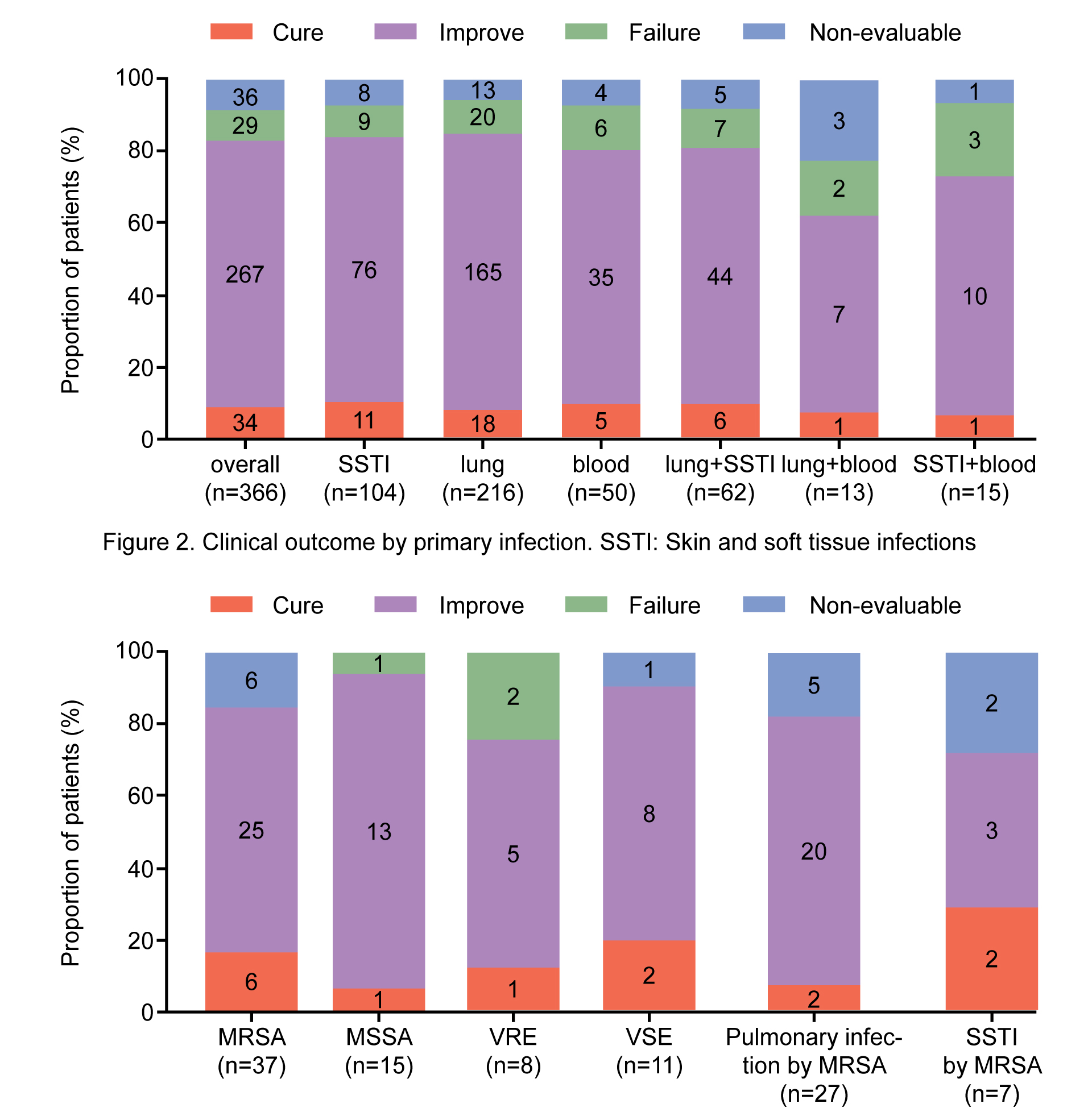
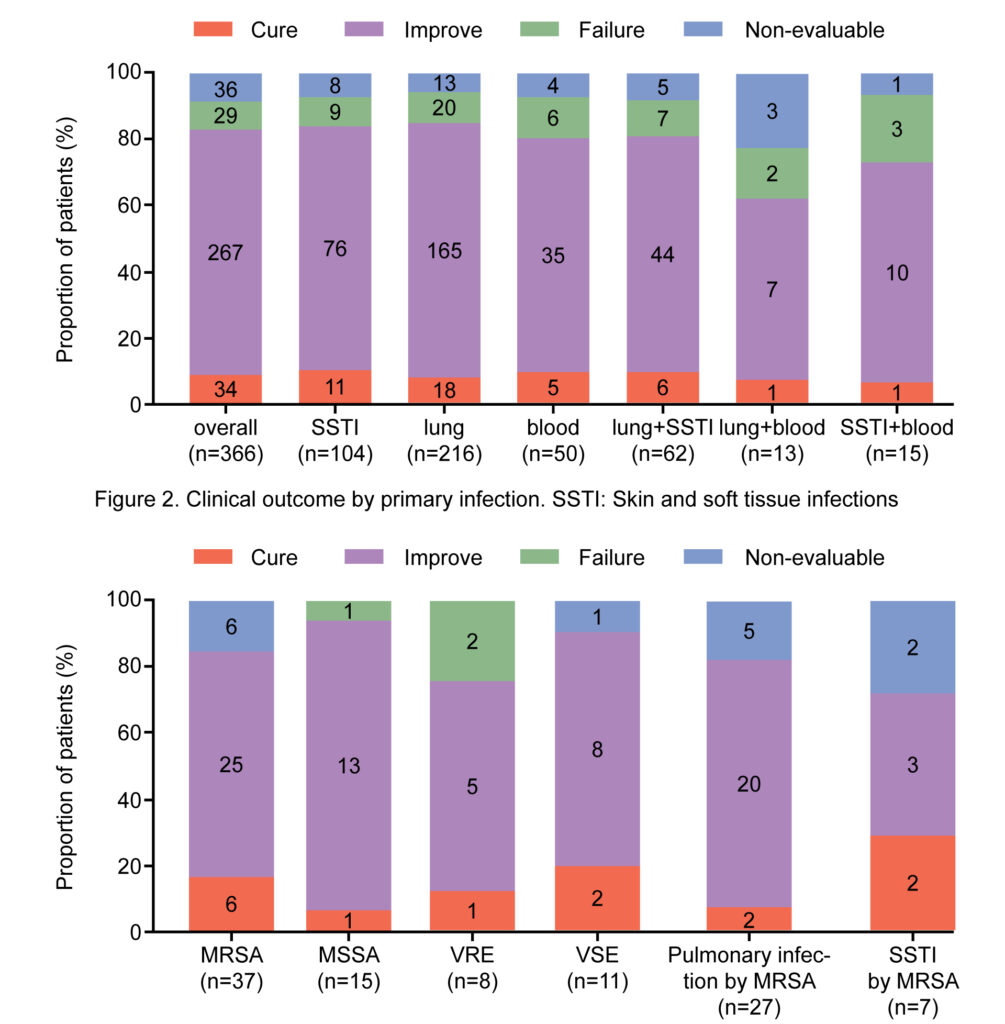
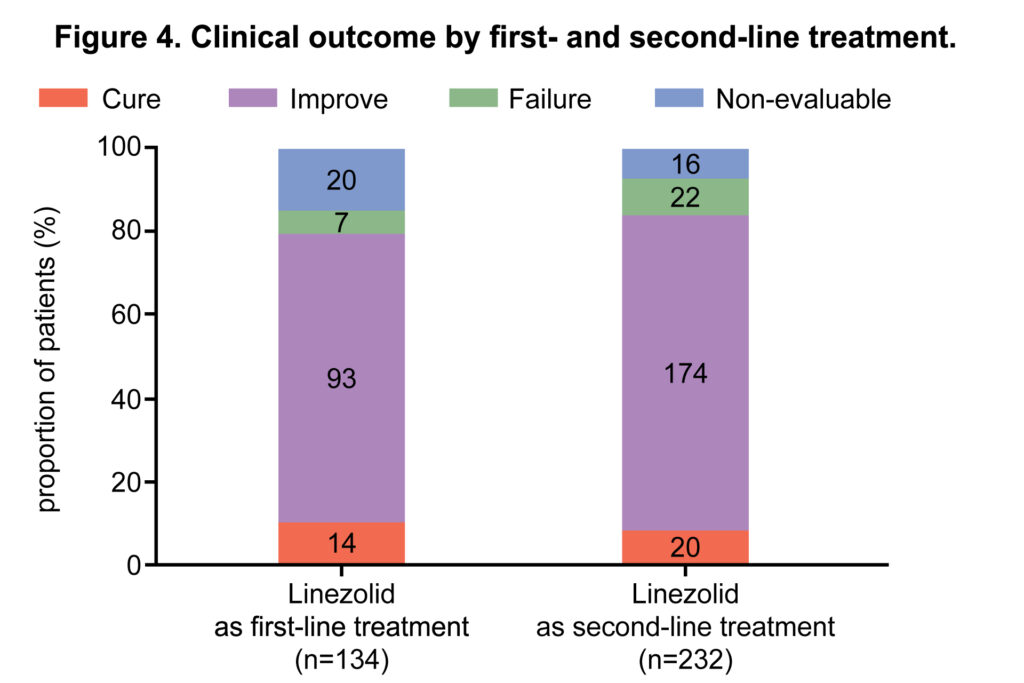
A total of 366 ICU patients who met the inclusion criteria were evaluated. Linezolid was used as second and first-line treatment in 232 (63.4%) and 134 (36.6%) patients, respectively. The most common isolated strain was Staphylococcus aureus (methicillin-resistant Staphylococcus aureus: n=37/119, 31.1%; methicillin-susceptible Staphylococcus aureus: n=15/119, 12.6%); this was followed by Enterococci (vancomycin-resistant Enterococci: n=8/119, 6.7%; vancomycin-susceptible Enterococci: n=11/119, 9.2%) and Streptococcus pneumoniae (multi drug resistant: n=4/119, 3.4%; non-multidrug resistant: n=2/119, 1.7%). The main infection sites where pathogens were detected included the lung (n=216/366, 59.6%), skin and soft tissue (n=104/366, 28.4%), and blood (n=50/366, 13.7%). Clinical success was achieved in 301 (82.2%) patients; 34 (9.3%) were cured and 267 (73.0%) improved; treatment failure and non-evaluable outcomes were observed in 29 (7.9%) in 36 (9.8%) patients, respectively. Linezolid-related adverse events were reported in 8 (2.2%) patients. No treatment-related serious adverse events were reported.
Conclusions:
The treatment of Gram-positive bacterial infections in the ICU is extremely important, and the selection of appropriate antibiotics based on patients’ clinical characteristics is the key to prognosis. Based on real-world results, linezolid has been found to be effective and safe in the treatment of Gram-positive bacterial infections in critically ill patients. Linezolid showed better clinical success in pulmonary infections or SSTIs caused by Staphylococcus aureus. Our results will provide intensivists with a reference for the selection of medication. However, due to the limitations of this study including those pertaining to sample size, clinicians will need to individually evaluate patient conditions before using linezolid in the clinic. In addition, they will need to be vigilant regarding possible side effects during administration.