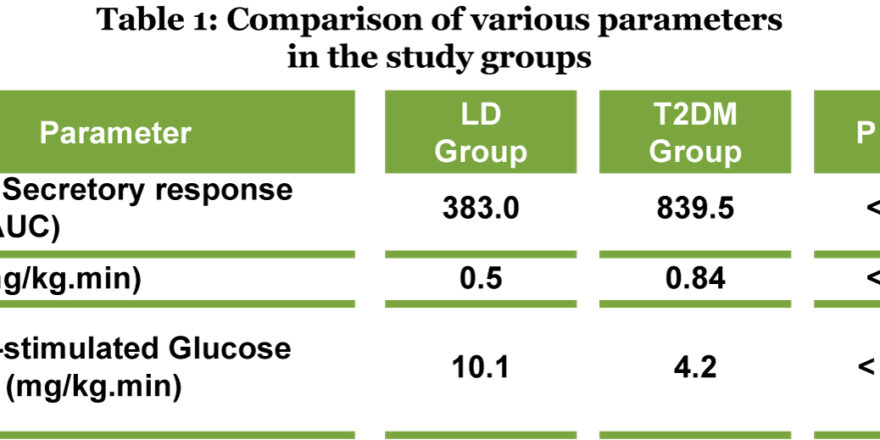
Ref: Michael Böhm, European Heart Journal (2022) 00, 1–12, https://doi.org/10.1093/eurheartj/ehac693
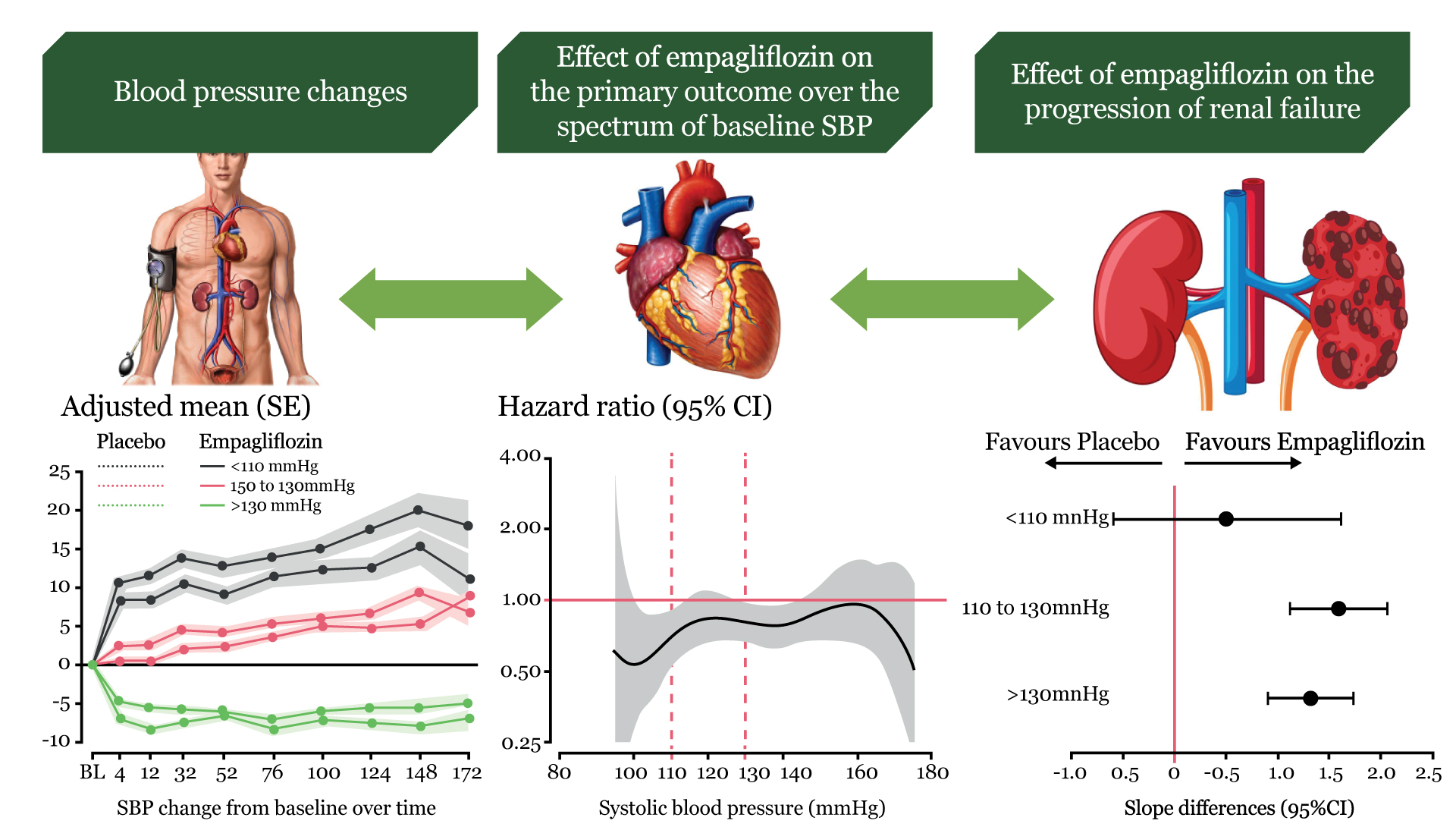
Empagliflozin reduced cardiovascular death and heart failure hospitalization in patients with preserved ejection fraction. Hypertension is the most common comorbidity and etiological trigger of heart failure with preserved ejection fraction (HFpEF) as pressure overload produces left ventricular hypertrophy, diastolic dysfunction, abnormal arterial-ventricular coupling, and other complications such as kidney failure. Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce systolic blood pressure (SBP) in patients with diabetes and hypertension, while in heart failure with reduced ejection fraction (HFrEF), only patients with a high baseline SBP had a significant and meaningful reduction. Registry data show an increase of heart failure outcomes and death for patients with SBP 140– 150 mmHg without differences between HFrEF and HFpEF. This U-shaped blood pressure (BP)-risk association might not be related to a causality rather than reflecting reverse causation as low BP selects patients with more advanced heart failure and frailty. In EMPEROR-Preserved (empagliflozin outcome trial in patients with chronic heart failure with preserved ejection fraction), we studied the effect of empagliflozin on SBP and its effects on heart failure outcomes and estimated glomerular filtration rate (eGFR) decline across baseline SBP levels in heart failure with ejection fraction >40%.
Methods and results:
The association of SBP and the treatment effects of empagliflozin in EMPEROR-Preserved (empagliflozin outcome trial in patients with chronic heart failure with preserved ejection fraction) was evaluated. Randomized patients (n=5988) were grouped according to SBP at baseline (130 mmHg, n=3118). The effect of empagliflozin on blood pressure, cardiovascular death or HF hospitalization (primary outcome), total HF hospitalizations, and rate of decline in estimated glomerular filtration rate was studied. Over a median of 26.2 months, the placebo-corrected decline was small and not significantly different across baseline SBP. On placebo, the risk of cardiovascular death or hospitalization for HF was 8.58 at >130 mmHg, 8.26 at 110–130 mmHg, and 11.59 events per 100 patient-years at 130 mmHg, P=0.08 vs. 110–130 mmHg). There was no evidence for baseline SBP moderating the effect of empagliflozin on risk of HF events (primary endpoint interaction P=0.69, recurrent HF hospitalizations interaction P=0.55). When comparing empagliflozin with placebo, SBP did not meaningfully associate with adverse events such as hypotension, volume depletion, and acute renal failure.
Discussion:
In EMPEROR-Preserved, there was a small placebo-corrected SBP decline by empagliflozin compared with placebo with an overall increase of SBP at low baseline SBP and a small drop of SBP at high baseline SBP on placebo and on empagliflozin possibly reflecting regression to the mean. In HFpEF, the outcome rates of the primary outcome were higher at low SBP ((<110 mmHg) but the treatment effect of empagliflozin on heart failure outcomes was not significantly related to baseline SBP with a similar risk reduction of heart failure hospitalization and cardiovascular death. Empagliflozin had minor effects on hypotension and volume depletion, while some fewer events were observed for acute renal failure (Structured Graphical Abstract).
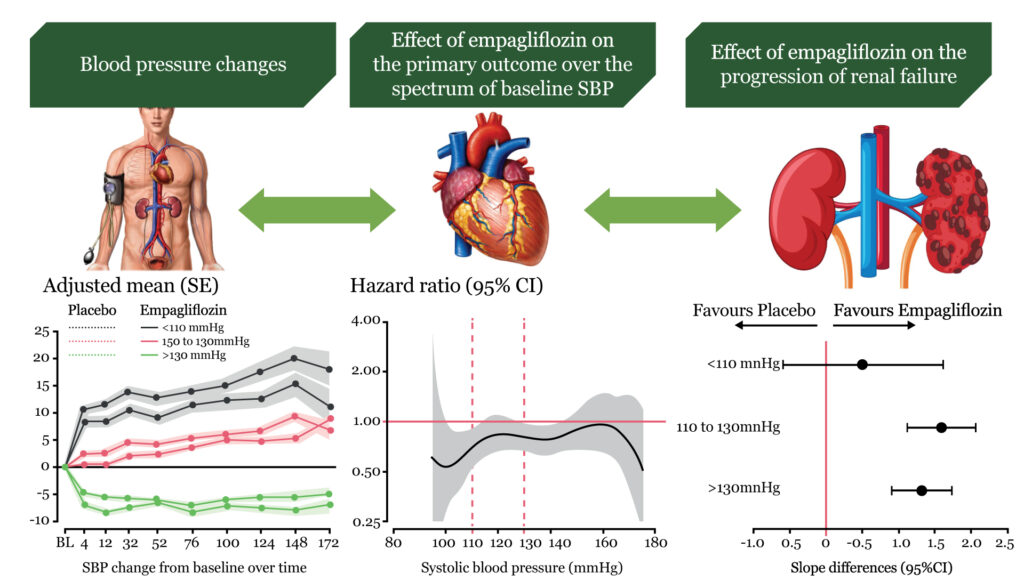


HFpEF is a heterogeneous condition with hypertension being one of the most prevalent and possible etiological factors promoting the progression of hypertrophy to failure with more patients presenting with a history of hypertension in HFpEF than in HFrEF. A higher prevalence of high BP in HFpEF compared with HFrEF is shown by the finding that 52.0% of the patients had an SBP of >130 mmHg and 7.6% of EMPEROR-Reduced 28.2% were at >130 mmHg and 24.2% had an SBP of <110 mmHg.8 Patients with heart failure are often undertreated when SBP is low, although they have a worse prognosis than those with a higher SBP. Interestingly, the U- and J-shaped association of SBP and DBP on mortality appears to be similar in HFrEF and HFpEF. Herein, we show that the primary outcome and cardiovascular death was increased in patients at SBP <110 mmHg compared with >110 mmHg. This finding is similar to previous HFrEF trials with treatment effects of SGLT2 inhibitors and sacubitril/valsartan being similar across SBP groups. We extended those findings to HFpEF patients by showing that the effect of empagliflozin is similar across baseline SBP groups in EMPEROR-Preserved.
In EMPEROR-Preserved, 90.6% of patients had a history of hypertension. Although high SBP is a major driver for the development of HFpEF, we still found a significant number with normal or low SBP. Nevertheless, the proportion of patients with low SBP <110 mmHg is lower than in HFrEF.8 A reduced myocardial systolic or diastolic function has been speculated to be involved in drops of SBP over time, previously termed ‘decapitated hypertension’, also observed in PARAGON-HF in HFpEF patients, and associated with increased HF events in HFrEF and HFpEF. Similar findings of an increased risk for cardiovascular outcomes at low SBP have also been shown in patients after myocardial infarction or stroke or known coronary artery disease. Nevertheless, in HFpEF as in HFrEF, SBP appears not to be an effect modifier of the cardio-renal effects of empagliflozin and, thus, might not contribute mechanistically to the treatment effect of empagliflozin.
As the effect of empagliflozin is maintained at SBP <110 mmHg, it is important that the protective effect does not come at a meaningfully increased cost of safety outcomes. There was only a slight increase of incident hypotension or volume depletion, while the incidence of acute renal failure was reduced. These observations are of clinical relevance as physicians are often reluctant to initiate treatment due to the fear of these adverse events at low SBP.
Conclusion:
In EMPEROR-Preserved, empagliflozin was effective and safe without SBP meaningfully moderating empagliflozin’s treatment effects. This analysis of EMPEROR-Preserved shows that empagliflozin can be used safely and effectively without blood pressure being a meaningful moderator of the drug benefit.